Ice crystals in ice cream
12 MINUTE READ The best ice creams in the world have a smooth and creamy texture. This creamy texture, primarily associated with a high fat content, is also determined by the average size of the ice crystals. Ice crystal size is governed by the mix formulation, as well as by factors relating to the freezing process. In this post, I'll be discussing the latter, which will include: residence time; the evaporation temperature of the refrigerant fluid; dasher speed; and draw temperature. Interspersed throughout this post will also be tips for optimising domestic ice cream machines.
The best ice creams in the world have a smooth and creamy texture. This creamy texture, primarily associated with a high fat content, is also determined by the average size of the ice crystals. Ice crystal size is governed by the mix formulation, as well as by factors relating to the freezing process. In this post, I'll be discussing the latter, which will include: residence time; the evaporation temperature of the refrigerant fluid; dasher speed; and draw temperature. Interspersed throughout this post will also be tips for optimising domestic ice cream machines.
1. ICE CRYSTALS IN ICE CREAM
Ice crystals range in size from about 1 to over 150 μm in diameter, with an average size of about 25 μm (Berger et al., 1972; Caldwell et al., 1992; Donhowe & Hartel, 1996; Hagiwara & Hartel, 1996; Hartel, 1996; Koxholt et al., 2000; Marshall et al, 2003; Sofjan & Hartel, 2004; Inoue et al., 2008; Kusumaatmaja, 2009). Small ice crystals, around 10 to 20 µm in size, give ice cream its smooth and creamy texture, whereas larger ice ice crystals, greater than 50 μm, impart a grainy texture (Marshall et al., 2003; Eisner et al, 2005; Drewett & Hartel, 2007).
To produce ice cream with the smallest possible ice crystals, it’s important to develop an understanding of ice formation (known as crystallisation) during the freezing of ice cream.
2. THE ICE CREAM FREEZING PROCESS
Ice cream is frozen in two stages, the first being a dynamic process where the mix is frozen in a scraped-service freezer (SSF) (ice cream machine) whilst being agitated to incorporate air, destabilise the fat, and form ice crystals. Upon exiting the SSF, the ice cream, at about -5°C to -6°C (23 to 21.2°F) and with a consistency similar to soft-serve ice cream, undergoes static freezing where it is hardened in a freezer without agitation until the core reaches a specified temperature, usually -18°C (-0.4°F). To read my discussion on the factors affecting ice crystal size during static freezing, please click here.
2.1. DYNAMIC FREEZING
Cook & Hartel (2010) argue that the dynamic freezing stage is arguably the most important step in creating ice cream because this is the only stage in which crystallisation occurs. During dynamic freezing, the ice cream mix is added to the SSF at between 0°C and 4°C (32°F and 39.2°F). As the refrigerant absorbs the heat in the mix, a layer of ice freezes to the wall of the cold barrel wall causing rapid nucleation, that is, the birth of small ice crystals (Hartel, 2001). The crystals that form at the cold barrel wall are then scraped off by the rotating dasher and scraper blades and dispersed into the centre of the barrel, where warmer mix temperatures cause some crystals to melt and others to grow and undergo recrystallisation. Recrystallisation is defined as “any change in number, size, shape, orientation or perfection of crystals following completion of initial solidification” (Fennema, 1973).
2.1.1. NUCLEATION
For smooth and creamy ice cream, it’s important to have a high rate of nucleation so as to form as many small ice crystals as possible (Hartel, 1996). The more ice crystals that are formed during dynamic freezing, the more will be preserved during static freezing, resulting in a smaller average crystal size and smoother texture (Cook & Hartel, 2010). Fewer crystals formed during dynamic freezing, or a lower rate of nucleation, results in coarse texture as these crystals eventually grow to a significantly larger size (Hartel, 1996).
2.1.2. GROWTH AND RECRYSTALLISATION
Cebula & Russell (1998) note that crystallisation during dynamic freezing can be divided into 2 zones: the wall region, where the temperature at the barrel wall is cold enough for nucleation to occur, and the bulk region, where warmer temperatures in the centre of the barrel mean that ice crystal growth and recrystallisation, also called ripening or coarsening, predominate. The greater the extent of growth and recrystallisation in the bulk region, the larger the ice crystals will be.
Russell et al. (1999) found that crystallisation during ice cream freezing is dominated by recrystallisation and growth and that these mechanisms appear to be more important than nucleation in determining the final crystal population. Minimising growth and recrystallisation is, therefore, of paramount importance.
3.FACTORS AFFECTING NUCLEATION, GROWTH, AND RECRYSTALLISATION
3.1. RESIDENCE TIME
Residence time (the length of time ice cream spends in the SSF) has a significant effect on the final ice crystal size distribution, with shorter residence times producing ice creams with smaller ice crystals due to a decline in recrystallisation (Russell et al., 1999; Koxholt et al., 2000; Goff & Hartel, 2013; Drewett & Hartel, 2007; Cook & Hartel, 2010).
A longer residence time means that ice cream is slower to reach its draw temperature (the temperature at which ice cream is extracted from the SSF) of around -5°C to -6°C (23°F to 21.2°F), which means that it spends more time in the bulk zone where warmer temperatures cause rapid recrystallisation. Donhowe & Hartel (1996) measured a recrystallisation rate at -5°C (23°F) of 42 μm/day. At this rate, a size increase of around 8 μm would be expected over a 10 minute period. This matches almost exactly the increase in crystal size observed by Russell et al. (1999) at a slightly different temperature of -4°C (24.8°F). Clearly, the longer ice cream remains within the SSF at temperatures where recrystallisation occurs very rapidly, the greater the extent of recrystallisation, and the larger the ice crystals.
Investigating the effect of draw temperature, dasher speed, and residence time on ice crystal size, Drewett & Hartel (2007) concluded that residence time had the greatest impact on final crystal size distribution, followed by drawing temperature and dasher speed.
3.2. EVAPORATION TEMPERATURE OF THE REFRIGERANT FLUID
Primary refrigerants (i.e. liquid ammonia or freon) are used in SSFs to provide temperatures in the range of -23°C to -29°C (-9.4°F to -20.2°F), with temperatures at the barrel wall being a few degrees warmer (Goff & Hartel, 2013). Decreasing the refrigerant temperature promotes rapid heat removal at the barrel wall. Rapid heat removal allows for faster ice nucleation rates, which results in smaller ice crystals due to the higher number of small crystals (Berger et al., 1972; Cook & Hartel, 2010; Arellano et al., 2012; Gonzalez-Ramirez, 2011).
Investigating ice crystal size in sorbet, Arellano et al. (2012) demonstrated that low refrigerant temperatures (up to -19.9°C (-3.82°F)) led to lower draw temperatures and a significant reduction in the ice crystal chord length. This was due to faster freezing, which caused faster formation of more ice crystals. Similarly, Gonzalez-Ramirez et al. (2011) showed an important reduction in ice crystal length as a function of a decreasing evaporation temperature.
3.2.1. BARREL WALL TEMPERATURE EFFECT ON RESIDENCE TIME
The barrel wall temperature has a direct effect on the cooling rate (the rate at which heat is removed from the ice cream mix), and therefore on residence time (Cook & Hartel, 2010). Lower wall temperatures can lower the bulk temperature of the ice cream faster, reducing residence time and improving the ice crystal size distribution (Russell et al., 1999; Drewett & Hartel, 2007).
3.2.2. TIP FOR DOMESTIC ICE CREAM MACHINES
If you’re using the Cuisinart ICE-30, or any other machine that requires the bowl to be frozen overnight, set your freezer’s temperature to as cold as it will go. I use the 'Super Freeze' function on my freezer to get the temperature down to around -30°C (-22°F) when freezing my ICE-30 bowl. Lower freezer temperatures will result in a lower bowl temperature, which will promote higher rates of nucleation and shorter residence times. If you’re using the Cuisinart ICE-100, Breville BCI600XL, Lello Musso Pola 5030, Lello Musso Lussino 4080, Whynter ICM-200LS, or any other machine with an in-built compressor, switch the compressor on and leave it running for about 15 minutes before you add your mix.
3.3. DASHER SPEED
During dynamic freezing, heat input from the rotating scraper blades, due to friction at the barrel wall and viscous dissipation, is significant, accounting for as much as 50% of the total heat removed by the refrigerant (Schwartzberg, 1990; Hartel, 1996; Russell et al., 1999). Increasing the dasher speed has been shown to cause an increase in the ice cream temperature, resulting in a significant increase in the average ice crystal size (Cebula & Russell, 1998; Russell et al., 1999). This likely occurs because the extra frictional heat generated by the blades melts many of the smallest crystals, resulting in a lower nucleation rate and the enhancement of recrystallisation (Donhowe & Hartel, 1996; Cook & Hartel, 2010). For this reason, dasher speeds are usually limited to 100-200 revolutions per minute (rpm) (Goff & Hartel, 2013).
3.4. EFFECT OF DASHER SPEED ON RESIDENCE TIME
Schwartzberg (1990) notes that the large amount of frictional heat inputted by higher dasher speeds will also slow the freezing process, resulting in longer residence times.
3.5. DRAW TEMPERATURE
Draw temperature has a significant influence on mean ice crystal size, with lower drawing temperatures generally resulting in smaller ice crystals (Arbuckle, 1986 Knoxholt et al., 2000; Drewett & Hartel, 2007). Hartel (1996) argues that of all the factors affecting ice crystallisation that can be controlled, the draw temperature is probably the most significant. Factors influencing draw temperature include the refrigerant temperature, heat transfer, residence time, and overrun.
3.5.1. REFRIGERANT TEMPERATURE EFFECT ON DRAW TEMPERATURE
Drewett & Hartel (2007) found that ice crystals were larger at draw temperatures from -3 to -6°C (26.6°F to 21.2°F). When the draw temperatures were colder than -6°C (21.2°F), the mean ice crystal size decreased. The researchers attributed the smaller ice crystal sizes to the lower refrigerant temperatures necessary to obtain lower draw temperatures. Figure 6 below, from Drewett & Hartel (2007), shows the effect of draw temperature on ice crystal size distribution.
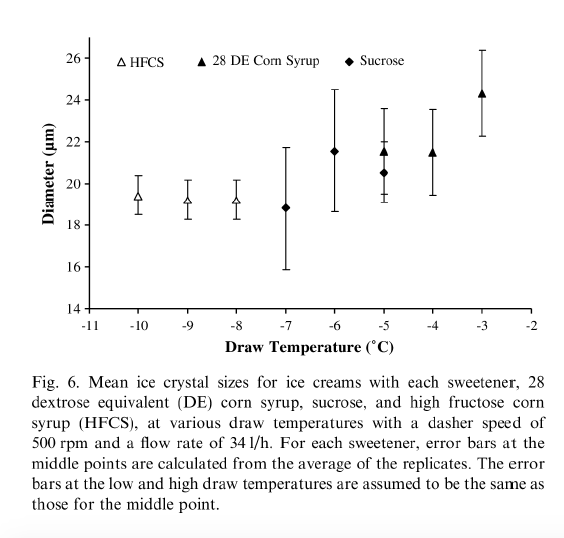 3.5.2. HEAT TRANSFER EFFECT ON DRAW TEMPERATURE
3.5.2. HEAT TRANSFER EFFECT ON DRAW TEMPERATURE
To attain low draw temperatures, heat transfer at the barrel wall must also be as efficient as possible. Because heat travels more slowly through ice than stainless steel, ice building up on the barrel wall acts as an insulator and slows heat transfer from the ice cream to the refrigerant. Efficient scraping, by keeping the blades sharp and close to the wall, ensures that heat transfer remains fast, giving smaller ice crystals (Marshall et al., 20003).
Ben Lakhdar et al., (2005) found that enlarging the gap between the blade extremity and the wall increased the internal heat transfer resistance, slowing heat transfer. This the researchers attributed to a permanent ice layer, which forms between the blade extremity and the wall when the gap between the blades and wall is large enough (3 mm). Where the gap is 1 mm, the ice layer is not strong enough and is periodically removed from the wall.
3.5.3. TIP FOR DOMESTIC ICE CREAM MACHINES
I use my thumb to push the main scraper blade on my Cuisinart ICE-30 against the side of the bowl during dynamic freezing. This prevents a build up of ice on the bowl wall, increasing heat transfer, promoting a higher rate of nucleation, and contributing to a reduction in the residence time. This does put more strain on the drive motor but I’ve found that after nearly 7 years of using this technique, my ICE-30 is still going strong. I wouldn't recommend this technique for machines where the drive motor is connected to, and turns, the scraper blades.
3.5.4. DASHER SPEED EFFECT ON DRAW TEMPERATURE
There appear to be conflicting reports on the effect of dasher speed on draw temperature. Arellano et al., (2012) found that an increase in dasher speed lead to a very slight increase in draw temperatures. Similarly, Gonzalez-Ramirez et al. (2011) reported that when dasher speed was varied from 600 to 900 rpm, a 1°C (1.8°F) increase in draw temperature, due to frictional energy transmitted to the ice cream, was observed. Conversely, Ben Lakhdar et al. (2005) showed that an increase in dasher speed led to an increase in the heat transfer at the barrel wall, producing lower draw temperatures.
3.5.5. RESIDENCE TIME EFFECT ON DRAW TEMPERATURE
Lower draw temperatures can also be attained through longer residence times. As previously noted, however, longer residence times mean that ice cream spends more time at temperatures where rapid growth and recrystallisation occur, resulting in larger ice crystals. Koxholt et al. (2000) note that the dynamic freezing step must account for competing phenomena as shorter residence times are needed to produce small ice crystals, but longer residence times give a lower draw temperature.
3.5.6. OVERRUN AND DRAW TEMPERATURE EFFECT ON ICE CRYSTAL SIZE
Inoue et al. (2008) found that the drawing temperature had the highest effect on mean ice crystal diameter, followed by the mix flow rate (which determines the average residence time), overrun, and dasher speed. The researchers found that when the drawing temperature was warmer than -5°C (23°F), mean ice crystal diameter was strongly dependent on the drawing temperature, with larger mean ice crystals reported at warmer draw temperatures. When the drawing temperature was colder than -5°C (23°F), however, not only the draw temperature, but also the overrun (the amount of air whipped into ice cream), influenced the mean ice crystal diameter.
The researchers noted that no significant differences were seen in the mean ice crystal diameter when the drawing temperatures were between -5°C and -6.5°C (23°F and 20.3°F) and the overrun was below 70%. At higher overruns, however, the mean ice crystal diameter was observed to be smaller. It was clearly shown that tiny ice crystals were formed when both the overrun and dasher speed were raised to their maximum (dasher speed 300 rpm and overrun 120%). Furthermore, the effect of frictional energy attributable to the increasing dasher speed on ice crystal size was never observed.
Smaller ice crystals as a result of higher dasher speeds reported by Inoue et al. (2008) is not, however, in agreement with the data reported by Russell et al. (1999), who showed that increasing the dasher speed causes an elevation in product temperature, which leads to the melting of small crystals and enhanced recrystallisation.
4. SUMMARY
Ice crystal size is a critical factor in the development of smooth and creamy ice cream. Creamy ice cream requires the majority of ice crystals to be small, around 10 to 20 µm in size. If many crystals are larger than this, the ice cream will be perceived as being coarse.
To produce small ice crystals during dynamic freezing, a high rate of nucleation, minimal growth, and minimal recrystallisation are required, with the latter two mechanisms being more important than the former in determining the final crystal population. Colder refrigerant temperatures and slower dasher speeds have been shown to promote higher rates of nucleation. Shorter residence times, lower dasher speeds, and lower draw temperatures have been shown to minimise growth and recrystallisation.
I hope this post helps. I'd love any feedback on how this post could be improved so do get in touch and say hi! All the best, Ruben :)
REFERENCES:
Arbuckle, W.S., 1986. Ice Cream. Chapman and Hall, London, 4th edition.
Arellano, M., Benkhelifa, H., Flick, D., Alvarez, G., 2012. Online ice crystal size measurements during sorbet freezing by means of the focused beam reflectance measurement (FBRM) technology. Influence of operating conditions. Journal of Food Engineering. 113(2). 351-359.
Ben Lakhdar, M., Cerecero, R., Alvarez, G., Guilpart, J., Flick, D., Lallemand, A., 2005. Heat transfer with freezing in a scraped surface heat exchanger. Applied Thermal Engineering. 25(1), 45–60.
Berger, K. G., Bullimore, B. K., White, G. W., and Wright, W. B., 1972. The structure of ice cream: part 1. Dairy Ind. 37(8):419–25.
Caldwell, K.B, Goff, H. D., Stanley, D. W., 1992. A low-temperature scanning electron-microscopy study of ice cream 1. Techniques and general microstructure. Food Struct. 11(1):1–9.
Cebula, D. J., Russell, A. B., 1998. Ice crystallization control in ice cream. In: BuchheimW, (ed). International Dairy Federation international ice cream symposium. Proceedings of the international symposium.
Cook, K. L. K., Hartel, R. W., 2010. Mechanisms of Ice Crystallisation in Ice Cream Production. Comprehensive Reviews in Food Science and Food Safety. 9 (2).
Donhowe, D. P., Hartel, R. W., 1996. Recrystallization of ice during bulk storage of ice cream. Int Dairy J. 6(11–12):1209–21.
Drewett, E. M., Hartel, R. W., 2007. Ice crystallisation in a scraped surface freezer. Journal of Food Engineering 78(3).
Eisner, M. D., Wildmoser, H., Windhab, E. J., 2005. Air cell microstructuring in a high-viscous ice cream matrix. Colloids Surf A. 263(1–3). 390–9.
Fennema, O. R., Powrie, W. D., Marth, E. H., 1973. Low Temperature Preservation of Foods and living Matter. USA: Marcel Dekker, Inc.
Goff, H. D., Hartel R. W., 2013. Ice Cream. Seventh Edition. New York Springer.
Gonzalez-Ramirez, J. E., Arellano, M., Leducq, D., Alvarez, G., Benkhelifa, H., Flick, D., 2011. Moments model for a continuous sorbet crystallization process. International Congress of Refrigeration.
Hagiwara, T., Hartel, R. W. 1996. Effect of sweetener, stabilizer, and storage temperature on ice recrystallization in ice cream. J Dairy Sci. 79(5):735–44.
Hartel, R. W., 1996. Ice crystallisation during the manufacture of ice cream. Trends in Food Science & Technology. 7(10).
Hartel, R. W., 2001. Crystallisation in foods. Gaithersburg, MD: Aspen Publishers.
Inoue, K., Ochi, H., Taketsuka, M., Saito H., Sakurai, K., Ichihashi, N., Iwatsuki, K., Kokubo, S., 2008. Modelling of the effect of freezer conditions on the principal constituent parameters of ice cream by using response surface methodology. Journal of Dairy Science. 91(5). 1722-32.
Koxholt, M., Eisenmann, B., Hinrichs, J., 2000. Effect of process parameters on the structure of ice cream. Bur Dairy Mag. 1:27-30.
Kusumaatmaja, W., 2009. Effects of mix pre-aeration and product recirculation on ice cream microstructure and sensory qualities [MSc thesis]. Madison, WI: University of Wisconsin - Madison. p.136.
Marshall, R. T., Goff, H. D., Hartel R. W., 2003. Ice cream. 6th ed. New York: Kluwer Academic/Plenum Publishers.
Russell, A. B., Cheney, P. E., Wantling, S. D., 1999. Influence of freezing conditions on ice crystallisation in ice cream. Journal of Food Engineering. 29.
Schwartzberg, H. G., 1990. Food freeze concentration. In: Rao, M. A., Schwartzberg, H. G., editors. Biotechnology and food process engineering. IFT basic symposium series. New York: Marcel Dekker. p 127–201.
Sofjan, R., P., Hartel, R. W., 2004. Effects of overrun on structural and physical characteristics of ice cream. International Dairy Journal. 14(3). 255-262.

